In this chapter, we will discuss the importance of taking accurate and precise measurements and recording detailed observations, both in chemistry or any other field. SI (International Standard) prefixes, base units, and derived units will be reviewed, and we will practice converting between different units of measurement.
In addition, the concept of significant figures will be introduced for the first time, and calculations involving significant figures will be performed. Finally, we will also review writing numbers in both scientific and standard notation. The measurement and calculation skills we learn in this chapter will be applied and used throughout the year.
There is a lot of math involved in the study of chemistry. Therefore, it is important for to review the metric system and some of the units we will be using this year.
Chemistry Notes Chapter 2 (Measurements & Calculations): 2-1 Scientific Method Scientific. Been selected for study during an experiment or observation Hypotheses Are. Lab 9 Answers.pdf; Palo Alto High; CHEMISTRY 10 - Spring 2015. Chemistry: Chapter 2 Measurements and Calculations. For multiplication or division, the answer can have no more significant figures than are in.
It goes without saying that chemistry uses the metric system. You need to stop thinking in terms of pounds, Fahrenheit, and gallons, and start thinking in terms of grams, Celsius, and liters. SI Base Units The SI system was adopted by the scientific community in 1960.
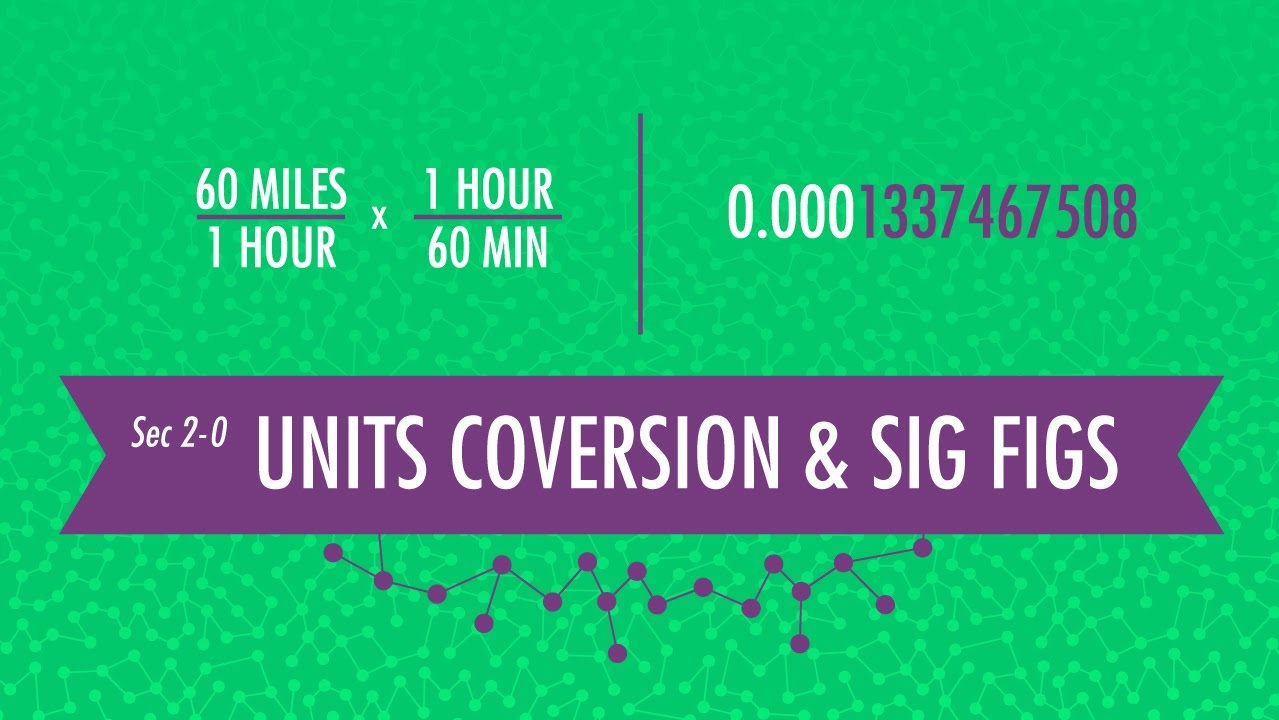
It is used as a common measurement system around the world. Below are the common base units used in chemistry. Converting Between Units Almost all of the math we will do this year will involve the need to convert between units. Therefore the sooner you master this skill the better off you'll be. We will be using the factor-label method to convert between units. In the factor-label method you take an equality and set it up in fraction form (conversion factor). You can do multiple conversions for one problem by lining up multiple conversion factors sequentially so that units on the top and bottom of neighboring conversion factors cancel.
For example, consider the equality of inches to centimeters (1.00 in = 2.54 cm). How many centimeters are in 5.00 inches? Significant Figures (Honors Only) In every measurement made, there is a degree of uncertainty. This is due to two reasons: humans are not perfect, and neither are the devices that we make to measure items. In any reported measurement, the numbers represent those know with certainty, and one (the last digit) that is uncertain. The 7 rules of significant figures: 1. All nonzero numbers (1-9) are ALWAYS significant.

For example: 1 234 = 4 significant figures 2. Zeros between nonzero numbers are significant For example: 1 004 = 4 significant figures 3.
Leading zeros to the left of the first nonzero number are NOT significant; they only show placement of the decimal point. For example: 0.0034 = 2 significant figures 4. Trailing zeros to the right of a decimal point are significant. For example: 0.00120 = 3 significant figures 5.
Modern Chemistry Measurements And Calculations
Trailing zeros to the left of a decimal point are NOT significant; they only show magnitude. For example: 12000 = 2 significant figures 6. All numbers in scientific notation are significant. For example: 3.040 x 10 3 = 4 significant figures 7.
Exact numbers have infinite significant figures. Exact numbers are numbers that are know with absolute certainty.

These typically include equalities (100 cm = 1 m) or things that have been counted (6 hockey players on the ice). Calculations with Significant Figures (Honors Only) When conducting calculations, the number of significant figures usually depends on the original measurement with the least amount of significant figures. There is a set of rules for addition and subtraction and a set of rules for multiplication and division. Addition/Subtraction Your answer should have the same number of decimal places as the original measurement with the fewest decimal places. For example,.
Measurements And Calculations Answers
202 (3 significant figures, zero decimal places) + 25.7 (3 significant figures, 1 decimal place) = 227.37, which should be rounded to 227 (3 significant figures, zero decimal places). Multiplication/Division In multiplication and division calculations, round the answer so as to have the same amount of significant figures as the original measurement with the least number of significant figures.
For example,. 6.0 (2 significant figures ) × 15.20 (4 significant figures) = 91.20, which should be rounded to 91 (2 significant figures). Scientific Notation In science we work with both very large and very small numbers. To simplify the use of these numbers in calculations, scientific notation is used. In scientific notation you have a coefficient that is greater than zero and less than 10 (0.